 ,
C. A. S. Hill2 and A. Gkaraveli3
,
C. A. S. Hill2 and A. Gkaraveli3| Holz als Roh- und Werkstoff European Journal of Wood and Wood Products |
| © Springer-Verlag 2004 |
| 10.1007/s00107-003-0448-8 |
A. N. Papadopoulos1  ,
C. A. S. Hill2 and A. Gkaraveli3
,
C. A. S. Hill2 and A. Gkaraveli3
| (1) | Technological Educational Institute of Karditsa, Department of Wood and Furniture Technology-Design, 43100 Karditsa, Greece |
| (2) | School of Agricultural and Forest Sciences, University of Wales, Bangor, Gwynedd LL57 2UW, Wales, UK |
| (3) | 3. Forest Authority of Magnesia Prefecture, 1 Xenofontos Str., 38333 Volos, Greece |
 |
A. N. Papadopoulos Email: antonios@1974@hotmail.com |
Published online: 12 February 2004
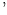 s with acetic anhydride.
s with acetic anhydride.Analyse des Quellungsverhaltens von chemisch modifiziertem Holz: Ein neuer Ansatz
Zusammenfassung Volumenänderungen aufgrund einer Modifikation mit Essig- und Capronsäureanhydrid beruhen auf dem Volumen, das die Rektanten einnehmen sowie dazu gehörigen Leerräumen. Letztere sind größer im Fall der Modifikation mit Capronsäure. Das mit Acetanhydrid modifizierte Holz nimmt pro Holzmasse weniger Wasser auf als das mit Capronsäure modifizierte, bezogen auf die prozentuale Massenzunahme (WPG). Eine nicht-lineare Beziehung ergab sich zwischen Wasseraufnahme und Volumenzunahme pro Gramm für nichtmodifiziertes korsiches Kiefernholz, wofür eine lineare Beziehung zu erwarten wäre. Dabei ist allerdings nicht das Volumen der Leerräume berücksichtigt. Wird dieses von der Sperrigkeit abgezogen, so ergibt sich tatsächlich eine lineare Beziehung nach der Modifikation mit Acetanhydrid, nicht aber mit Capronanhydrid. Es scheint also, daß das Modell der Leerräume eine vernünftige Erklärung liefert für das unterschiedliche Quellverhalten, das an korsischen Kiefern-Splintholz beobachtet wurde.
Various studies of the relationship between the swelling of the material due to modification have been published. In studies of the acylation of spruce, maple and balsa, it has been found that the degree of swelling of the substrate exhibits a proportional relationship with the degree of substitution, up to an acetyl content of 16–18% (Stamm and Tarkow 1947). Furthermore, the volume degree due to modification, has been found to be equal to the volume of the acetyl groups in the wood (Stamm and Tarkow 1947; Rowell and Ellis 1978).
More recently, this latter finding has been disputed, where it has been found that for Corsican pine modified wood, a volume increase larger than theoretically predicted is obtained (Hill and Jones 1996; Papadopoulos 2001).
The volumetric swelling of wood due to esterification, has been studied, and the effect of such modification upon the dynamic mechanical properties of wood determined (Nakano 1994). In this work, it was considered that volume increase of the wood was the sum of volume occupied by chemically bonded reagent, plus a void volume created in the cell wall polymeric network around the adduct.
When wood takes up moisture into the cell wall, the walls swell volumetrically in proportion to the volume of the water absorbed (Skaar 1988). This is based on the assumptions that the cell lumina is constant in size and that there are no voids in the cell wall and therefore, water simply adds its volume to that of dry wood.
The first assumption relies on modification either causing an increase in swelling (due to saturation) into the cell lumina or a reduction in the way in which the lumen size increases due to water swelling. Cell lumina in some wood species have been found to enlarge or shrink when wood is saturated and swells, though where this occurs it is often only by a small amount, and negligible compared to the swelling of the cell wall (Stamm 1964; Siau 1995). It was assumed therefore that no such changed occurred. Any change in dimension due to water swell was considered to be solely due to the swelling of the wood cell wall.
It was the aim of this paper to test the validity of the second assumption. For this reason, wood was modified with two linear chain carboxylic acid anhydrides, namely acetic and hexanoic.
Sapwood samples of dimension 20 mm×20 mm×5 mm (radial×tangential×longitudinal) were cut from freshly-felled kiln dried Corsican pine. Samples were carefully smoothed with sandpaper to remove loosely adhering fibres, then placed in a Soxhlet extractor for solvent extraction using toluene/methanol/acetone (4:1:1 by volume) for eight hours. Samples were dried in an oven for 8 h at 105°C. Samples were removed from the oven, transferred to a vacuum desiccator and allowed to cool to ambient temperature over silica gel. Prior to reaction, each sample was weighed on a four figure balance and the dimensions determined using a micrometer (accurate to±0.01 mm). Samples (five replicates) were then vacuum impregnated with dry pyridine (over KOH) for one hour, then transferred to a flask containing pyridine set in an oil bath at 100°C. Samples were allowed to equilibrate in the hot pyridine for one hour. After heating for one hour, the sample batch was transferred to a round bottom flask containing a one molar solution of the anhydride in pyridine set in an oil bath at 100°C. Samples were added at various time intervals so as to give a range of weight percent gains (reaction periods from 15 min to 7 h). At the end of the reaction period, the flask was removed from the oil bath, the hot reagent decanted off and ice cold acetone added to quench the reaction. Samples were kept in the acetone for 1 h, before being transferred to the Soxhlet apparatus for solvent extraction, as previously detailed. Samples were then oven dried at 105°C for 8 h, weight gain due to reaction recorded and dimensions taken.
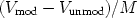
|
The method for controlling relative humidity is described by Stamm (1964), has been widely used and was selected for being simple, economical and reasonably precise. Test samples were kept above saturated solutions of various salts in containers stored in a controlled temperature room set at 20°C (variation±1°C). Pure water results in the saturated vapour pressure corresponding to 100% relative humidity. The addition of a solute to water reduces its vapour pressure in proportion to its mole fraction in the case of diluted solutions. When a saturated solution at a controlled temperature is used, a constant relative humidity is maintained (Siau 1995).
|
Salt |
Relative humidity (%) |
|---|---|
|
Potassium nitrate (KNO3) |
93 |
|
Sodium chloride (NaCl) |
76 |
|
Sodium dichromate (Na2Cr2O7) |
55 |
|
Potassium carbonate (K2CO3) |
44 |
|
Potassium acetate (CH3COOK) |
23 |
|
Lithium chloride (LiCl) |
12 |
Selected wpg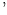 s of oven dry wood samples were placed in
the containers above saturated salt solutions. They were left to equilibrate for
4 weeks and then weighed once a week, using a four-place analytical balance
until it became obvious that no significant weight change had occurred since the
last weight was recorded (and EMC had been attained). Equilibrium moisture
content was reached within 6 weeks for all but the two highest humidities,
which required longer exposure times. Furthermore, it was observed that at each
relative humidity, the time required for the samples to attain EMC increased as
the molecular size of the adduct increased, i.e. samples modified with acetic
anhydride attained EMC quicker than those modified with hexanoic anhydride, at
comparable wpg
s of oven dry wood samples were placed in
the containers above saturated salt solutions. They were left to equilibrate for
4 weeks and then weighed once a week, using a four-place analytical balance
until it became obvious that no significant weight change had occurred since the
last weight was recorded (and EMC had been attained). Equilibrium moisture
content was reached within 6 weeks for all but the two highest humidities,
which required longer exposure times. Furthermore, it was observed that at each
relative humidity, the time required for the samples to attain EMC increased as
the molecular size of the adduct increased, i.e. samples modified with acetic
anhydride attained EMC quicker than those modified with hexanoic anhydride, at
comparable wpg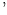 s.
s.
 s is obtained. This variation in molar
volume in the wood, with extent of modification is illustrated in Fig. 1.
The lines represent best fit curves to the data points. Both modificants showed
the same behaviour, in that larger molar volumes were found at lower levels of
substitution, with an asymptotic decrease to a stable value at higher wpg
s is obtained. This variation in molar
volume in the wood, with extent of modification is illustrated in Fig. 1.
The lines represent best fit curves to the data points. Both modificants showed
the same behaviour, in that larger molar volumes were found at lower levels of
substitution, with an asymptotic decrease to a stable value at higher wpg s. Thus,
it is apparent that if the volume increase observed in the wood samples has
contributions both from the reagent volume (Vi), and a
void volume (Vf) (Nakano 1988),
then the void volume is larger at lower levels of substitutions. This was
commented previously (Hill and Jones 1999).
s. Thus,
it is apparent that if the volume increase observed in the wood samples has
contributions both from the reagent volume (Vi), and a
void volume (Vf) (Nakano 1988),
then the void volume is larger at lower levels of substitutions. This was
commented previously (Hill and Jones 1999).
The determination of the apparent void volume created by the acyl groups within the wood cell wall polymeric network requires the use of theoretically calculated volumes for the attached acyl groups. These values are reported in the literature (Nakano 1988) and have been also used by Hill and Jones (1999). Those values not reported were obtained by extrapolation.
|
R |
V |
Vi |
Vf |
|---|---|---|---|
|
CH3 |
45 |
27.2 |
17.8 |
|
C5H11 |
115 |
68.4 |
46.6 |
 s (ca. 5–6%) a non-linear relationship is
obtained; however at higher wpg
s (ca. 5–6%) a non-linear relationship is
obtained; however at higher wpg s the relationship is linear. A linear
relationship was also reported by Risi and Arseneau (1957)
for acetic and phthalic anhydrides, though no explanation appears to have been
offered. This is further discussed later.
s the relationship is linear. A linear
relationship was also reported by Risi and Arseneau (1957)
for acetic and phthalic anhydrides, though no explanation appears to have been
offered. This is further discussed later.
The non-linear relationship between EMC and volumetric swelling
obtained at low wpg s (ca. 5–6%) probably indicates a
non-homogenous distribution of adduct at low levels of modification. It maybe
also due to the larger void volumes at low wpg
s (ca. 5–6%) probably indicates a
non-homogenous distribution of adduct at low levels of modification. It maybe
also due to the larger void volumes at low wpg s, which would depress volumetric
swelling at low emc
s, which would depress volumetric
swelling at low emc s. As a result, when these voids were filled
with water, the expected swelling was observed.
s. As a result, when these voids were filled
with water, the expected swelling was observed.

|
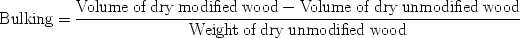
|
From this, it can be seen that for both chemicals a non-linear relationship is obtained. If it is assumed that the volume available in the cell wall for water decreases as a result of volume occupied by adduct, then a linear relationship between the two values would be expected. Thus, as more volume in the cell wall is occupied by adduct, there is less volume available for water.
On closer inspection, the results depicted in Fig. 4
indicate that at low wpg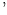 s (ca. 5–6%) more water is accommodated in
the cell wall in acetic anhydride modified wood than in hexanoic anhydride
modified wood. This indicates a shielding or masking effect with hexanoic
anhydride modified wood (Papadopoulos and Hill). It appears therefore, that the
adduct of the larger molecular size hexanoic anhydride, reacts with one hydroxyl
group but covers some of the adjacent ones, providing a physical barrier. This
barrier prevents the water vapour molecules from reaching some of the unreacted
sites, that is, it shields sites. Hence, the effect of the adduct covering
reacted and unreacted sites may overshadow the effect of direct chemical bonding
to the sites. At intermediate wpg
s (ca. 5–6%) more water is accommodated in
the cell wall in acetic anhydride modified wood than in hexanoic anhydride
modified wood. This indicates a shielding or masking effect with hexanoic
anhydride modified wood (Papadopoulos and Hill). It appears therefore, that the
adduct of the larger molecular size hexanoic anhydride, reacts with one hydroxyl
group but covers some of the adjacent ones, providing a physical barrier. This
barrier prevents the water vapour molecules from reaching some of the unreacted
sites, that is, it shields sites. Hence, the effect of the adduct covering
reacted and unreacted sites may overshadow the effect of direct chemical bonding
to the sites. At intermediate wpg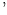 s (ca. 16%) both acetic and hexanoic
anhydride modified wood accommodated the same amount of water, as it can be seen
from Fig. 4.
At higher wpg
s (ca. 16%) both acetic and hexanoic
anhydride modified wood accommodated the same amount of water, as it can be seen
from Fig. 4.
At higher wpg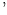 s (ca. 22.5%, although there is no data
available for hexanoic anhydride, and therefore an approximate value has been
obtained as indicated from the dotted line in Fig. 4)
less water was accommodated by acetic anhydride modified wood, the opposite to
what was observed at low levels of modification. This may be due to damage of
the cell wall in wood modified with hexanoic anhydride. As a consequence, new
sorption sites become available and extra space is created in the wood cell
wall.
s (ca. 22.5%, although there is no data
available for hexanoic anhydride, and therefore an approximate value has been
obtained as indicated from the dotted line in Fig. 4)
less water was accommodated by acetic anhydride modified wood, the opposite to
what was observed at low levels of modification. This may be due to damage of
the cell wall in wood modified with hexanoic anhydride. As a consequence, new
sorption sites become available and extra space is created in the wood cell
wall.
It seems therefore that the free volume model, offers a reasonable
explanation of the differences in swelling recorded for Corsican pine sapwood
modifying to varying wpg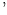 s with acetic anhydride. A wider range of
weight gains, a larger number of replicates, and even swelling measurement
during desorption would be required in a comprehensive study of this
phenomenon.
s with acetic anhydride. A wider range of
weight gains, a larger number of replicates, and even swelling measurement
during desorption would be required in a comprehensive study of this
phenomenon.
Volume changes due to modification with acetic and hexanoic
anhydride are due to the volume occupied by the reagent and an associated void
volume. The void volume is larger at low levels of modification, and larger in
hexanoic anhydride modified wood. Less weight of water per gm of unmodified wood
was accommodated by acetic anhydride modified wood than by hexanoic modified
wood, at equivalent wpg. A non-linear relationship was found between weight of
water per gm of unmodified Corsican pine wood and bulking, whereas a linear
relationship would be predicted. However, this takes no account of void volume.
When the value of void volume is deducted from the bulking a linear relationship
was indeed obtained with acetic anhydride, but not with hexanoic anhydride
modified Corsican pine. It seems therefore that the free volume model introduced
offers a reasonable explanation of the differences in swelling recorded for
Corsican pine sapwood modifying to varying wpg s with acetic anhydride. A wider
range of weight gains, a larger number of replicates, and even swelling
measurement during desorption would be required in a comprehensive study of this
phenomenon.
s with acetic anhydride. A wider
range of weight gains, a larger number of replicates, and even swelling
measurement during desorption would be required in a comprehensive study of this
phenomenon.