
| Holz als Roh- und Werkstoff European Journal of Wood and Wood Products |
| © Springer-Verlag 2005 |
| 10.1007/s00107-004-0551-5 |
| (1) | Department of Wood and Furniture, Technological Educational Institute of Karditsa, Technology-Design, 43100 Karditsa, Greece |
 |
Antonios N. Papadopoulos Email: antonios1974@hotmail.com |
Published online: 15 February 2005
Keywords Chemical modification - Esterification - Hailwood-Horrobin model - Sorption - Greek wood species
Sorptionsisothermen von zwei veresterten griechischen Laubhölzern
Zusammenfassung Zwei griechische Laubhölzer (Ulmus montana und Acer pseudoplatanus) wurden mit Acet- und Maleinanhydrid verestert und ihr Feuchte-Sorptionsverhalten untsersucht. Die Sorptionsisothermen der unbehandelten und chemisch behandelten Proben wurden mittels des Hailwood-Horrobin-Modells analysiert. Es zeigte sich, dass die Veresterung sowohl die polymolekulare als auch die monomolekulare Sorption beeinflusst. Die Behandlung mit Acetanhydrid war bei vergleichbarem Massenzuwachs effektiver als mit Maleinanhydrid bezüglich des Erniedrigens der hygroskopischen Eigenschaft, was wahrscheinlich mit den starken Esterbindungen zwischen Holz und Acetanhydrid zusammenhängt. Die Gleichgewichtsfeuchten waren bei vergleichbaren Gewichtszunahmen für beide Holzarten und beide Soptionstypen identisch, und zwar nicht nur für die unbehandelten, sondern auch für die modifizierten Proben.
The fibrous nature of wood has made it one of the most appropriate and versatile raw materials for a variety of uses. However, two properties restrict its much wider use: dimensional changes when subjected to fluctuating humidity, and susceptibility to biodegradation by microorganisms. The varying moisture content of wood results in dimensional and conformational instability, which can compromise the performance of other materials combined with wood, such as adhesives and surface coatings. Until relatively recently, these shortcomings were addressed by impregnating wood with appropriate hydrophobes (Stamm 1964; Kumar 1994). It has now been demonstrated that wood may be modified chemically so that selected properties are enhanced in a more or less permanent fashion (Rowell 1983; Hill and Papadopoulos 2002).
It has been shown that the dimensional stability of wood can be effectively improved by esterification with anhydrides (Rowell et al. 1988, Papadopoulos and Hill 2003). There is limited work reported on the water vapour sorptive properties of such modified woods. A number of authors have investigated the sorption isotherms of acetylated wood specimens at only one level of substitution (Risi and Arseneau 1957; Spalt 1958; Popper and Bariska 1972; Yasuda et al. 1995). Although the effect on overall stabilisation in response to liquid water soaking is well documented (Stamm 1964; Rowell 1983; Hill and Jones 1996), there is little evidence of how sorption is influenced by esterification with anhydrides. Recently, a comprehensive investigation into the water sorptive properties and into the effect of molecular size of the substituent group upon the sorption of water vapour of softwood modified with linear chain carboxylic acid anhydrides was carried out, using the Hailwood–Horrobin model (Papadopoulos and Hill 2003). It was concluded that the reduction in total, polymolecular and monomolecular adsorption produced by the linear chain anhydrides is primarily determined by the volume of adduct deposited in the cell wall (bulking) rather than by the number of hydroxyl groups which have been substituted.
In this study, water adsorption behaviour of two widely used Greek hardwoods, namely elm (Ulmus montana) and maple wood (Acer pseudoplatanus), esterified by acetic and maleic anhydride was investigated. Esterified wood was analysed by FTIR spectroscopic technique to study the changes in intensity of hydroxyl groups of cell wall polymers during the reaction with anhydrides. Adsorption isotherm was obtained to assess the hygroscopicity of esterified wood using the Hailwood–Horrobin sorption theory.
Sapwood samples of dimension 20×20×5 mm (radial×tangential×longitudinal) were cut from freshly felled kiln dried elm and maple. Samples were carefully smoothed with sandpaper to remove loosely adhering fibres, then placed in a Soxhlet extractor for solvent extraction using toluene/methanol/acetone (4:1:1 by volume) for 8 h and subsequently dried in an oven for 8 h at 105°C. Samples were removed from the oven, transferred to a vacuum desiccator and allowed to cool to ambient temperature over silica gel. Prior to reaction, each sample was weighed on a four-figure balance. Samples (30 replicates) were then vacuum impregnated with pyridine (dried over KOH) for 1 h, then transferred to a flask containing pyridine set in an oil bath at 100°C. Samples were allowed to equilibrate in the hot pyridine for 1 h. After heating for 1 h, the sample batch was transferred to a round bottom flask containing a one molar solution of the anhydride (acetic or maleic) in pyridine set in an oil bath at 100°C. At the end of the reaction period (5 h), the flask was removed from the oil bath, the hot reagent decanted off and ice cold acetone added to quench the reaction. Samples were kept in the acetone for 1 h, before being transferred to the Soxhlet apparatus for solvent extraction, as previously detailed. Samples were then oven dried at 105°C for 8 h and weight gain due to reaction recorded.
For Infra-red (IR) analysis, the treated samples were ground up by using a microdismembrator (20.000 rpm for 6 min). The fibre flour was then mixed with oven dry potassium bromide (KBr) powder (the fibre flour/KBr ratio was 1:100) and placed in a vibratory ball mill capsule. The mixture was ground for about 2 min. The ground mixture was then transferred to a press and the bolts of press screwed down. The bolts were tightened with a spanner to press the disk. After a few minutes, the bolts were loosen and removed. The press was placed directly into a sample beam of a Mattson FTIR spectrometer, Nicolet 750, series II.
|
Salt |
RH (%) |
|---|---|
|
Potassium nitrate (KNO3) |
93 |
|
Sodium chloride (NaCl) |
76 |
|
Sodium dichromate (Na2Cr2O7) |
55 |
|
Potassium carbonate (K2CO3) |
44 |
|
Potassium acetate (CH3COOK) |
23 |
|
Lithium chloride (LiCl) |
12 |
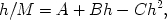 |
(1) |
![$$ A = \frac{W} {{18}}\left[ {\frac{1} {{K_2 (K_1 + 1)}}} \right], $$](2005_19.files/s00107-004-0551-5etEqu2.gif)
|
(2) |
![$$ B = \left( {\frac{W} {{1,800}}} \right)\left[ {\frac{{K_1 - 1}} {{K_1 + 1}}} \right], $$](2005_19.files/s00107-004-0551-5etEqu3.gif)
|
(3) |
![$$ C = \left( {\frac{W} {{180,000}}} \right)\left[ {\frac{{K_1 K_2 }} {{K_1 + 1}}} \right], $$](2005_19.files/s00107-004-0551-5etEqu4.gif)
|
(4) |
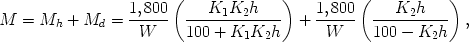
|
(5) |
|
Reagent |
WPG |
A |
B |
C |
K1 |
K2 |
Wo |
R2 |
|---|---|---|---|---|---|---|---|---|
|
Maple wood | ||||||||
|
Control |
0 |
3.22 |
11.46 |
10.57 |
5.68 |
0.76 |
294.3 |
0.992 |
|
Acetic |
15.3 |
7.73 |
13.38 |
13.88 |
3.37 |
0.72 |
443.9 |
0.851 |
|
Maleic |
16.3 |
5.58 |
13.18 |
12.83 |
4.18 |
0.74 |
386.0 |
0.822 |
|
Elm wood | ||||||||
|
Control |
0 |
3.58 |
10.78 |
10.27 |
4.95 |
0.76 |
292.0 |
0.981 |
|
Acetic |
14.1 |
8.21 |
12.95 |
14.64 |
3.06 |
0.76 |
458.3 |
0.884 |
|
Maleic |
15.9 |
5.99 |
12.47 |
12.84 |
3.75 |
0.75 |
387.3 |
0.844 |
As defined above, the constant K2 expresses the activity of dissolved water per unit relative vapour pressure. According to Okoh and Skaar (1980), its value should be unity if it has the same activity as liquid water. The K2 values vary approximately between 0.72 and 0.76, indicating that the dissolved water shows a lower activity than the liquid water. This suggests that the freedom of motion of water in the cell wall micropores (dissolved water) is not the same as that in liquid water.
|
Reagent |
WPG |
Reduction in hygroscopicity (%) | ||
|---|---|---|---|---|
|
|
|
Total |
Polymolecular |
Monomolecular |
|
Maple wood | ||||
|
Acetic |
15.3 |
41.7 |
41.3 |
42.5 |
|
Maleic |
16.3 |
29.4 |
29.4 |
29.4 |
|
Elm wood | ||||
|
Acetic |
15.3 |
38 |
35.8 |
44 |
|
Maleic |
16.3 |
27.1 |
26.1 |
29.8 |
The efficacy of modified wood with different anhydrides in reducing hygroscopicity has been the subject of many studies. A comprehensive investigation into the effect of molecular size of the substituent group of softwood modified with linear chain carboxylic acid anhydrides, namely acetic, propionic, butyric, valeric, hexanoic, upon the sorption of water vapour has been performed (Papadopoulos and Hill 2003). Analysis of the sorption isotherms, using the Hailwood–Horrobin model, at comparable weight percentage gain revealed that the five anhydrides used show similar effectiveness in both total, polymolecular and monomolecular sorption, despite the substantial difference in the proportion of hydroxyl groups reacted. It was concluded that the reduction in total, polymolecular and monomolecular sorption produced by the linear chain anhydrides is primarily determined by the volume of adduct deposited in the cell wall (bulking) rather than by the number of hydroxyl groups which have been substituted. The sorption properties of modified white fir with acetic and phthalic anhydride were measured by fitting isotherms to sorption data using the BET and Hailwood–Horrobin models (Popper and Bariska 1972). It was found that the reaction with acetic anhydride significantly reduced monomolecular adsorption, as the hydrophilic hydroxyl groups were replaced. In contrast, wood modified with phthalic anhydride gave monomolecular adsorption isotherms similar to untreated wood. This was attributed to the hydrophilic acid hydroxyl introduced during reaction with phthalic anhydride. Similar observation was also made by Chauhan et al. (2001) in rubber wood. In this case, not much difference was observed in the behaviour of maleic and phthalic anhydride treated wood.
The sorption isotherms for untreated and chemically modified wood were analysed using the Hailwood–Horrobin model. The experimental analysis of the sorption isotherms showed that esterification affects the total, polymolecular and monomolecular sorption. Acetic anhydride treatment was found more effective in reducing the hygroscopicity of wood compared to maleic anhydride treatment at comparable weight percentage gain, reflecting probably the strong ester bonds between acetic anhydride and wood. Identical e.m.c values were attained in both types of sorption for maple and elm wood, at equivalent WPG, not only for the unmodified samples but for the modified ones as well.